Advertisement
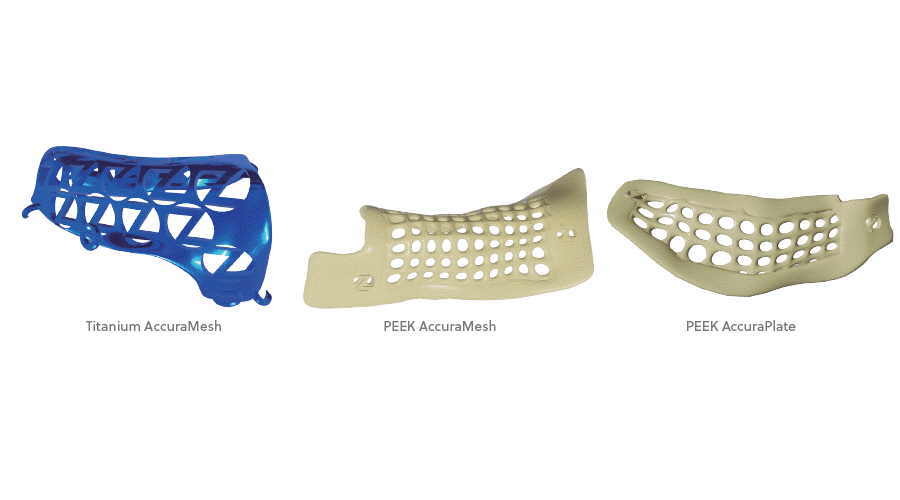
AccuraMesh and AccuraPlate
Guided Bone Regeneration
Key Benefit:
AccuraMesh and AccuraPlate products are designed using a fully digital workflow. Data from 3D medical imaging devices combined with modern Computer-Aided Design (CAD) software and state-of-the-art Computer-Aided Manufacturing (CAM) processes result in high quality customized medical devices for guided bone regeneration procedures. (1)
Clinical Advantages
• AccuraMesh and AccuraPlate are CAD/CAM manufactured to fit precisely to the defect site (2)
• 2 material options available, surgical grade PEEK and Titanium (Titanium for AccuraMesh only)
• Pre-planned screw positions for reliable fixation
• Sterile packaged (ETO sterilized): 10-6 Sterility assurance level (3-5)
• Additional manual adjustment of the bone bed or of the Accura products is seldomly required (2, 6)
• Reduced surgery time and morbidity (2,7)
AccuraPlate Is Typically used In:
• Regeneration of horizontal bone defects (5)
AccuraMesh Is Typically used In:
• Regeneration of horizontal and/or vertical bone defects (1,3,4)
1. Cruz N. et al. Materials (2020) 13:2177. 2. Lehman H. et al. Int J Oral Maxillofac Implants (2014) 29:e259-64. 3. Titanium AccuraMesh IFU latest revision. 4. PEEK AccuraMesh IFU latest revision. 5. PEEK AccuraPlate IFU latest revision. 6. Parthasarathy J. Ann Maxillofac Surg (2014) 4:9-18. 7. El Chaar E. et al. Int J Periodontics Restorative Dent (2019) 39:491-500. 8. Data on file with ResDevMed
Guided Bone Regeneration
Key Benefit:
AccuraMesh and AccuraPlate products are designed using a fully digital workflow. Data from 3D medical imaging devices combined with modern Computer-Aided Design (CAD) software and state-of-the-art Computer-Aided Manufacturing (CAM) processes result in high quality customized medical devices for guided bone regeneration procedures. (1)
Clinical Advantages
• AccuraMesh and AccuraPlate are CAD/CAM manufactured to fit precisely to the defect site (2)
• 2 material options available, surgical grade PEEK and Titanium (Titanium for AccuraMesh only)
• Pre-planned screw positions for reliable fixation
• Sterile packaged (ETO sterilized): 10-6 Sterility assurance level (3-5)
• Additional manual adjustment of the bone bed or of the Accura products is seldomly required (2, 6)
• Reduced surgery time and morbidity (2,7)
AccuraPlate Is Typically used In:
• Regeneration of horizontal bone defects (5)
AccuraMesh Is Typically used In:
• Regeneration of horizontal and/or vertical bone defects (1,3,4)
1. Cruz N. et al. Materials (2020) 13:2177. 2. Lehman H. et al. Int J Oral Maxillofac Implants (2014) 29:e259-64. 3. Titanium AccuraMesh IFU latest revision. 4. PEEK AccuraMesh IFU latest revision. 5. PEEK AccuraPlate IFU latest revision. 6. Parthasarathy J. Ann Maxillofac Surg (2014) 4:9-18. 7. El Chaar E. et al. Int J Periodontics Restorative Dent (2019) 39:491-500. 8. Data on file with ResDevMed

Wilhelm-Wagenfeld-Str. 28
80807 München
Germany
80807 München
Germany
more information
Hall 4.1 | D020 E021

